Nature Paper Online!
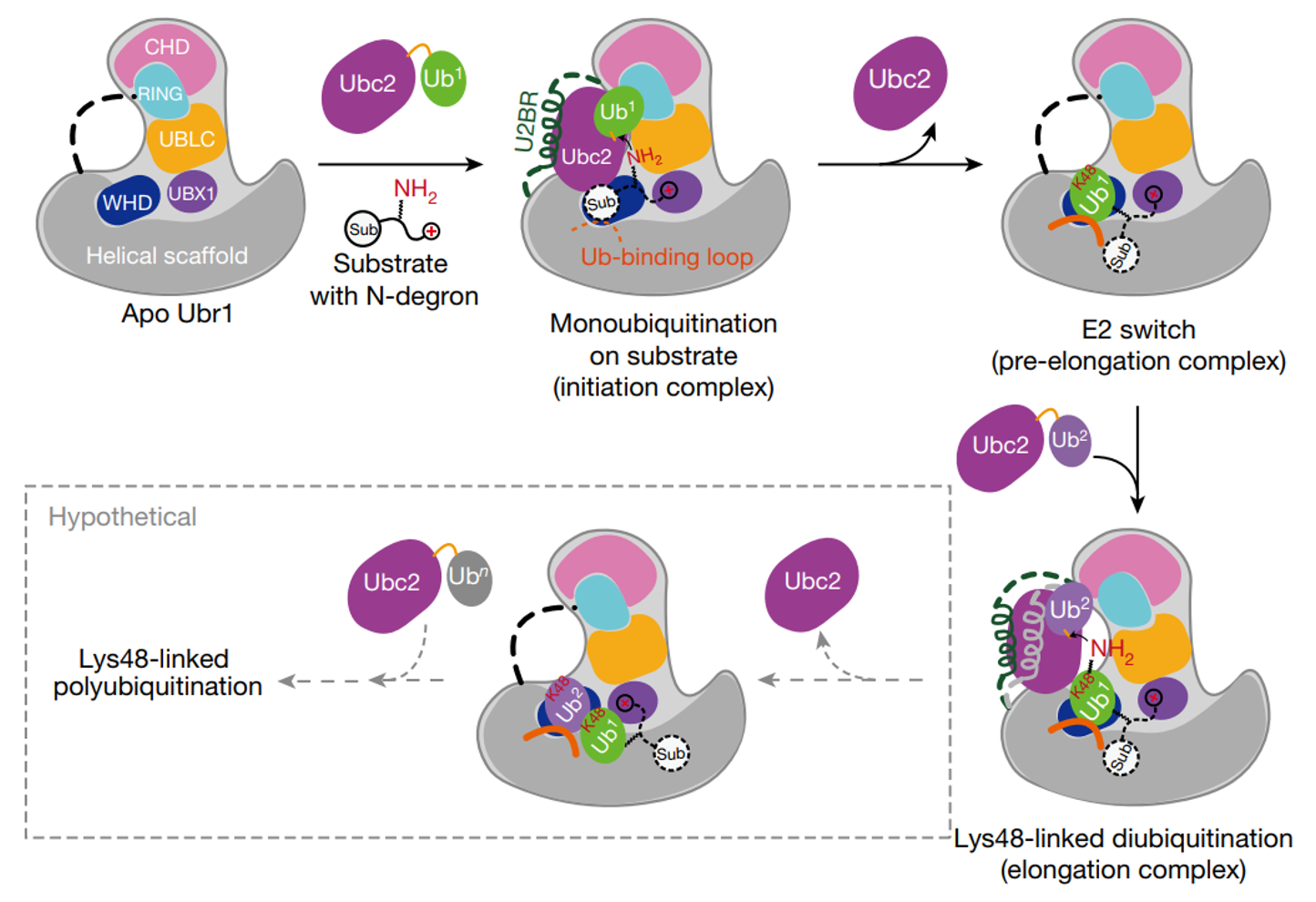
Our paper Structural insights into Ubr1 mediated N-dengron polyubiquitination is online. In this study, we used chemical biology methods to capture multiple intermediate states and determined the complex structures of yeast Ubr1, Ubc2, ubiquitin, and N-degron. This is the first time that the full-length structure of Ubr1, a single-subunit E3 ligase of ~2000 residues, is determined. Our structures revealed how Ubr1 initiates the ubiquitination reaction on the N-degron peptide and elongate the ubiquitin chain in a linkage specific manner. They showed that E3s can also contribute to the linkage specificity of ubiqutin chains, and provided a mechanistic understanding of Ubr1 mediated polyubiquitination. Historically, Ubr1 was the first genetically cloned E3 ligase (Bartel, et al., 1990). It is invovled in the N-degron pathway. We are very pround of our structures and findings!
Also check out the highlights at UChicago BSD Research News and Chinese media. Thank all the wonderful collaborators at the Liu Lab in Tsinghua University and congratulations to Man Pan, Qingyun Zheng, Tian Wang, and Lujun Liang!
